High-selectivity graphene membranes enhance CO₂ capture efficiency
16/07/2024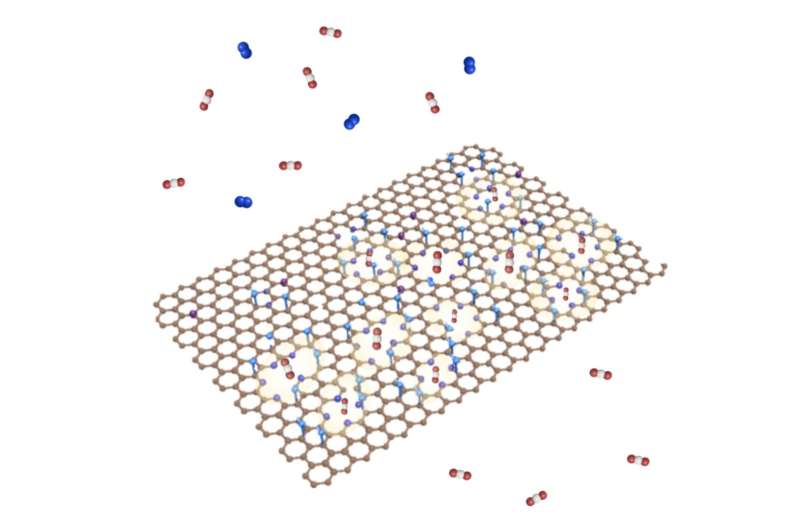
A schematic of porous graphene hosting pyridinic N (shown as purple spheres) at the pore edges. The resulting membrane is highly selective to CO2. Credit: Dr. Kuang-Jung Hsu.
Reducing carbon dioxide (CO₂) emissions is a crucial step towards mitigating climate change and protecting the environment on Earth. One proposed technology for reducing CO₂ emissions, particularly from power plants and industrial establishments, is carbon capture.
Carbon capture entails the separation of CO₂ from mixed gas emissions and capturing it to prevent its release into the air. One approach to doing this is to use special membranes that serve as selective "barriers," allowing CO₂ to pass through them and absorbing it, while blocking the passage of other gases.
So far, developing high-performance and low-cost membranes that can capture CO₂ has proved challenging. This has significantly reduced the potential of these solutions for real-world applications.
Researchers at École Polytechnique Fédérale de Lausanne (EPFL) recently introduced new graphene membranes that could enable high performance carbon capture. These membranes, presented in a paper published in Nature Energy, incorporate pyridinic nitrogen at their pore edges, which facilitates the binding of CO₂ to its pores.
"We were looking to advance the separation performance of graphene membranes," Kumar Varoon Agrawal, corresponding author for the paper, told Phys.org. "We had done a lot of work in increasing porosity in graphene, improving size distribution of pores, and adding polymer groups to the pore to improve CO2/N2 selectivity as well as obtain high CO2 permeance. However, we either obtained high permeance or high selectivity but not both."
After reviewing past literature and conducting their own studies aimed at developing membranes for carbon capture, Agrawal and his colleagues realized that graphene-based membranes exhibiting both high selectivity and permeance were still lacking. To move toward the development of these solutions, they set out to devise a method that would improve the binding of CO₂ to graphene pores.
The method they proposed entails exposing ammonia to oxidized single-layer graphene at room temperature. This process was found to incorporate pyridinic nitrogen at the edges of the membrane's pores, which boosts the binding of these pores with CO2.
"We introduced atomic N at the graphene pore in the form of pyridinic N," Agrawal said. "This form of N has a high affinity to CO2. This approach is beneficial because the graphene lattice remains atom-thin and allows us to obtain both high selectivity and permeance."
The researchers found that their method led to membranes with a promising average CO2/N2 separation factor of 53 and an average CO2 permeance of 10,420 from a stream containing 20 vol% CO2. For a diluted CO2 stream with a volume % of ~1, the membrane attained separation factors above 1,000.
"We could carry out pyridinic N incorporation by a simple method, simply soaking porous graphene in ammonia," Agrawal said. "We noticed that this led to a remarkable improvement in CO2/N2 selectivity while maintaining exceptional permeance. Also, this led to extremely high CO2/N2 selectivity for dilute CO2 feed, above 1,000, which is extremely attractive."
The graphene membranes developed by Agrawal and his colleagues and the approach used to fabricate them could open new opportunities for the large-scale implementation of carbon capture techniques. The researchers are now working on scaling up the membranes and simplifying their fabrication by roll-to-roll synthesis, to facilitate their future commercialization.
Source: https://tinyurl.com/3yffnkp7 via Phys.Org

